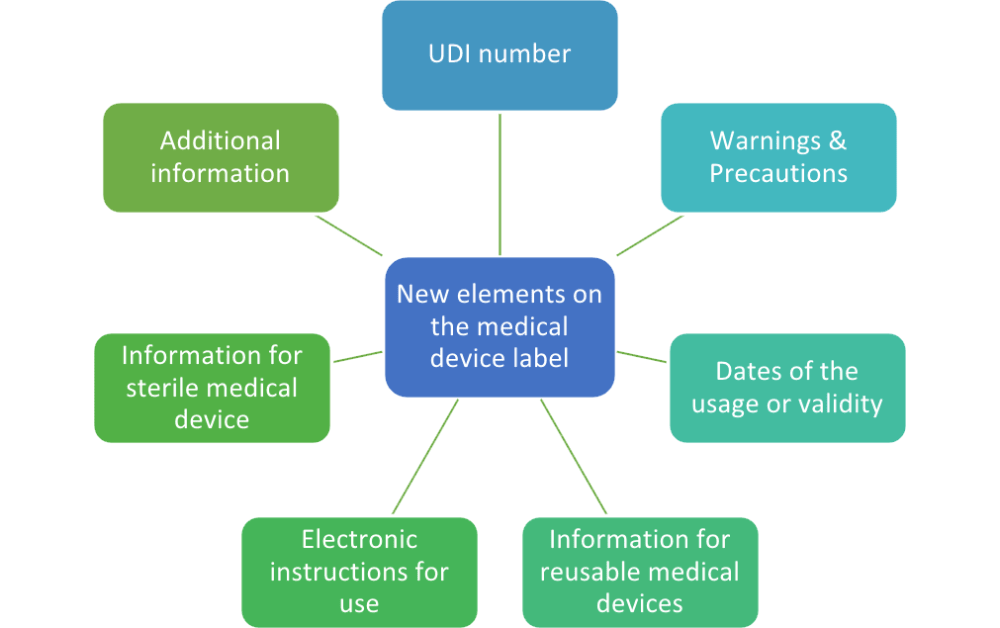
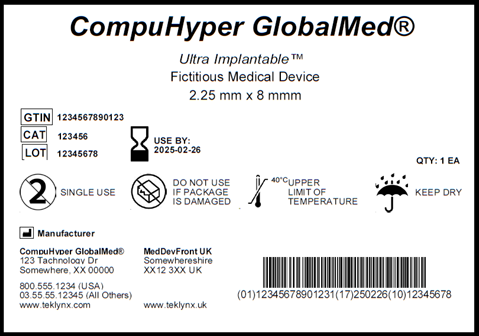
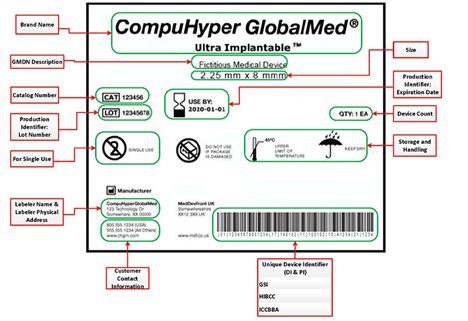
EU Medical Device Regulation: manufacturers' responsibilities - Medical Technology | Issue 8 | July 2018

EU MDR - Medical Device Labeling Changes & Challenges - Regulatory, Clinical Consulting Services to Biopharma & Medical Device Companies